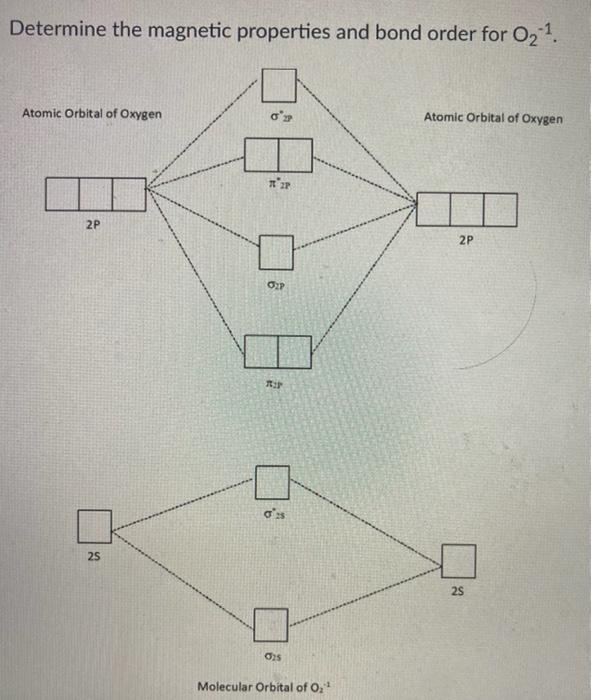
Compare the relative stability of the following species and indicate their magnetic properties : O2, O^+2, O2^ - (superoxide) and O2^2 - (peroxide).

Energy level diagram of O2 and find out its bond order and magnetic properties - Chemistry - Chemical Bonding and Molecular Structure - 13264653 | Meritnation.com
Write the Electronic configuration, Energy level diagram for the molecular orbitals of Oxygen molecule (O2). - Sarthaks eConnect | Largest Online Education Community

Write the molecular orbital electronic distribution of oxygen specify its bond order and magnetic - Brainly.in
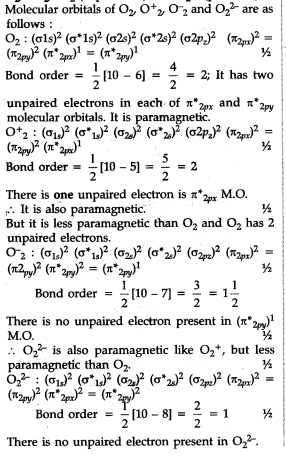
Compare the relative stability of the following species and indicate their magnetic properties - CBSE Class 11 Chemistry - Learn CBSE Forum
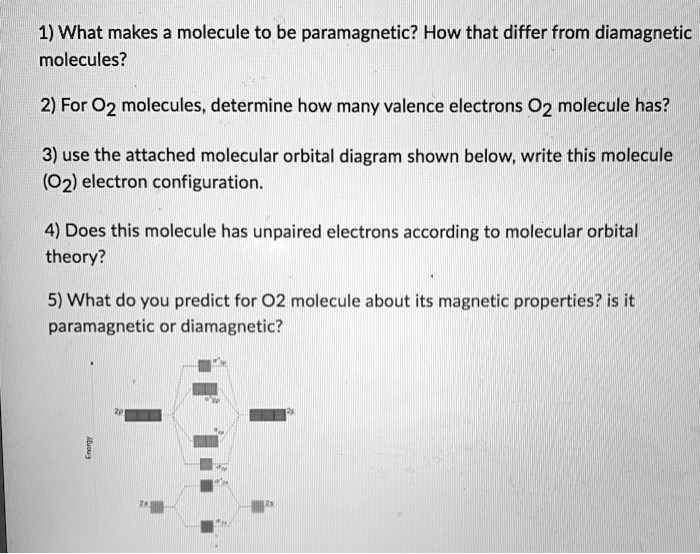
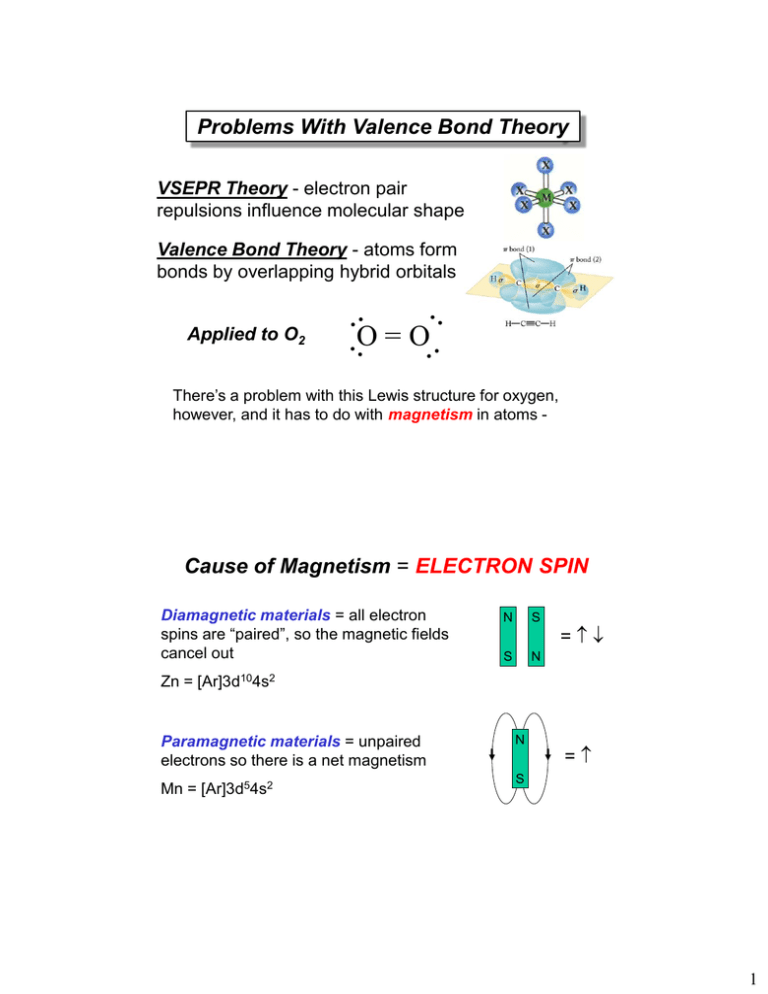
SOLVED:1) What makes molecule to be paramagnetic? How that differ from diamagnetic molecules? 2) For 02 molecules, determine how many valence electrons 02 molecule has? 3) use the attached molecular orbital diagram

Draw and write the molecular configuration of nitrogen molecule. Claculate its bond order and magnetic property.
Compare the relative stability of the following species and indicate their magnetic properties; - Sarthaks eConnect | Largest Online Education Community

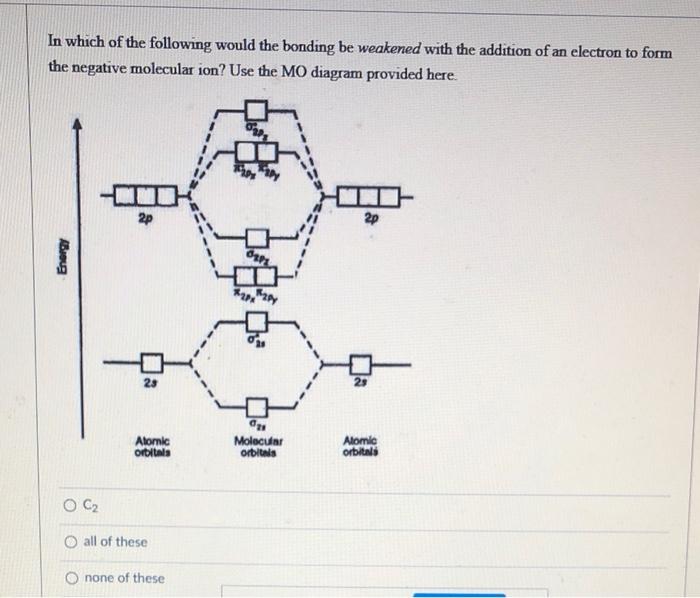
Based on molecular orbital theory compare the stability and magnetic property of o2+ and o2- - Brainly.in

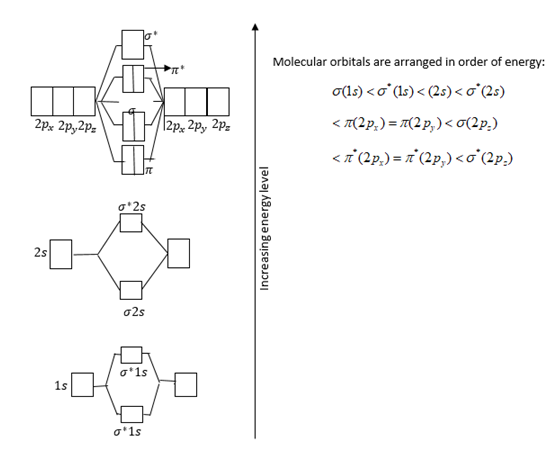
Molecular Orbital Theory Edward A. Mottel Department of Chemistry Rose-Hulman Institute of Technology. - ppt download

Effect of O2 adsorption on magnetic properties of oxygen-deficient ZnO nanoparticles - ScienceDirect
Magnetic Properties of the Oxygen Molecule in Solid Oxygen‐Argon Mixtures: Journal of Applied Physics: Vol 40, No 3

Write the molecular orbital electron distribution of oxygen `(O_(2))` Specify its bond order and - YouTube

Compare the relative stability of the following species and indicate their magnetic properties: O2,O2+,O2