- Hydrogen peroxide is decomposed by the rough surfaces of glass, alkali oxides present in it, and light
- Therefore, to prevent its decomposition, H2O2 is usually stored in coloured paraffin wax-coated plastic or Teflon bottles
indeed, Why is hydrogen peroxide placed in a brown bottle rather than in a transparent one? The brown bottle in your medicine cabinet is a bulwark against those two catalysts Light cannot penetrate the tint of the brown bottle, preventing oxidation, which can result in an increase in temperature As peroxide breaks down, it gets hotter As a result, the compound’s decomposition rate accelerates
Why is hydrogen peroxide not stored in glass bottles?
(i)- Hydrogen peroxide should not be stored in glass bottles because the glass has a rough surface and these have alkali oxides present So when this is exposed to light, it causes the decomposition of hydrogen peroxide and causes an explosion
Similarity, How hydrogen peroxide is stored and why? Hydrogen peroxide topical solution should be stored in tight, light-resistant containers at 15-30 deg C To ensure greater stability, the inside surfaces of containers should be as free as possible from rough points since these promote decomposition
Why chemicals are stored in brown bottles?
Chemicals are vulnerable to molds and bacteria Brown Bottles maintain the shape of a chemical and avoid any reactions visible light can make chemicals decompose When the bottle is darkened, the light converts to heat when it hits the bottle
What happens when hydrogen peroxide is exposed to light?
Like all other peroxides, hydrogen peroxide (H2O2) also consists of a relatively weaker O−O bond, which is susceptible for light or heat In the presence of light (the UV light from the sun catalyzes the reaction), H2O2 spontaneously decomposes into water and oxygen
Why are hydrogen peroxide and silver nitrate kept in coloured bottles?
– Silver nitrate decomposes on reacting with light into silver – Hydrogen peroxide reacts with light and decomposes to form water – So, they are kept in dark coloured bottles to prevent them from reacting with light – If they are kept in colourless bottles, they will undergo decomposition reaction
How do you store hydrogen peroxide?
Hydrogen peroxide topical solution should be stored in tight, light-resistant containers at 15-30 deg C To ensure greater stability, the inside surfaces of containers should be as free as possible from rough points since these promote decomposition
Does hydrogen peroxide react with glass?
You may also notice that peroxide never comes in a clear, glass container That’s because glass bottles may contain dissolved alkali metal ions that can react with the solution
How long is hydrogen peroxide good for once opened?
Hydrogen peroxide You need to replace hydrogen peroxide six months after opening it, but it will last for three years unopened To test whether it is still effective, you can pour it in to the sink and see if it fizzes and bubbles If it does, it’s still good Expired hydrogen peroxide is ineffective but not harmful
What should hydrogen peroxide not be stored with?
Hydrogen Peroxide Hydrogen peroxide should never be stored with copper, chromium, iron, most metals or their salts, alcohols, acetone, organic materials, aniline, nitromethane, flammable liquids, ammonia or oxidizing gases
What container can hold hydrogen peroxide?
Plastic tanks are suitable for up to 50% hydrogen peroxide provided they are made of correct polymeric material Example plastics are polypropylene, polytetrafluoroethylene, polyvinylidene fluoride such as Solvay SOLEF®, and a co-polymer of vinylidene fluoride and hexafluoro propylene such as VITON®
How should hydrogen peroxide stored?
Store hydrogen peroxide in the original vented container, upright, in a cool, ventilated area where it is protected from damage, or in bulk storage tanks made from approved materials of construction Do not store other chemicals, fuels, or combustible materials near hydrogen peroxide
Does hydrogen peroxide react with plastic?
H2O2 is a strong oxidizer I wouldn’t even dare try it on anything metal On plastic, it would perfectly be fine as long as it’s not the industrial 35% grade

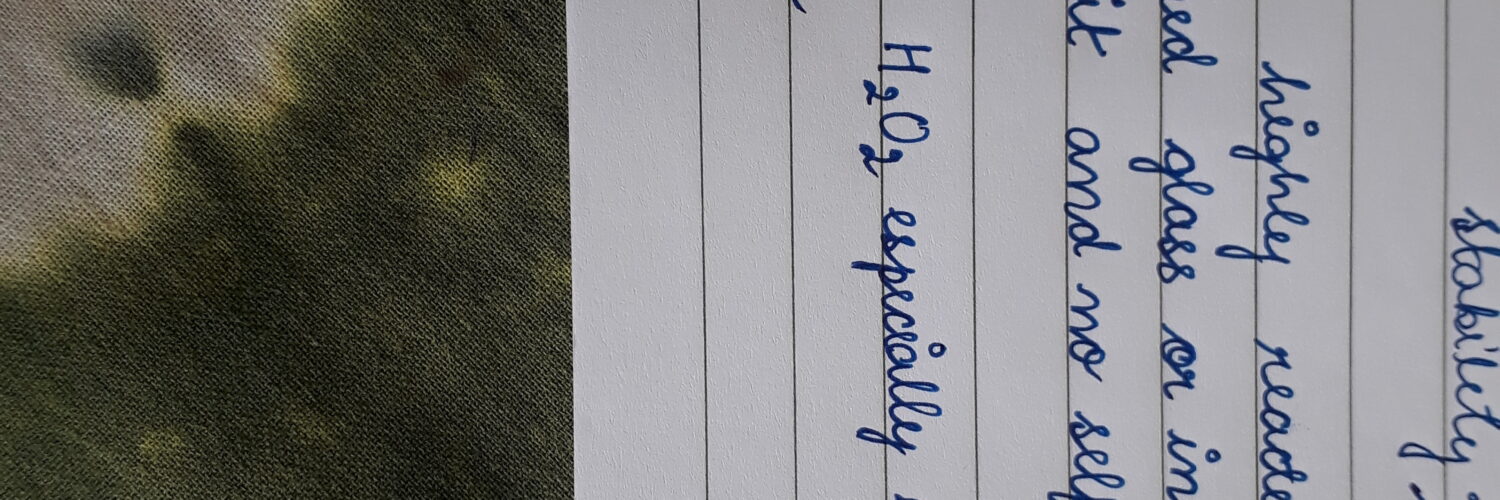

Add comment